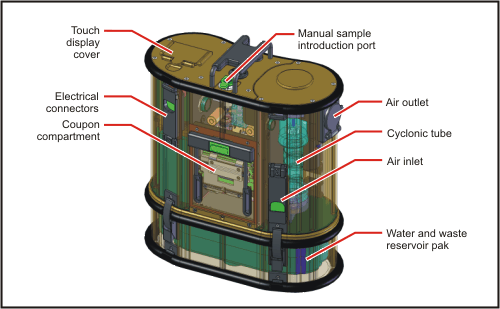
During my dissertation defense presentation a couple of weeks ago, I explained that one of the advantages of any fluorescence-based biological or chemical detection system would be the ability to scale down such a system to a portable and deployable size. My evidence for this was the mention of two biosensing detection systems commercialized by Research International: the Raptor and the Biohawk. Both of these systems are, for lack of a better word, awesome. Both systems use what are known as sandwich immunoassays to detect the target analytes. And both systems are capable of detecting multiple analytes simultaneously. The sandwich immunoassay used in these sensors is an antibody-based detection method in which a primary antibody specific to a target compound is adsorbed onto a glass waveguide, similar to an optical fiber. If the target compound is present in a sample, it will bind selectively to the primary antibody. After exposure to a sample, a solution containing a secondary antibody that is labeled with a fluorescent dye is flown over the waveguide. If the target compound is bound to the primary antibody, the secondary antibody will also bind to it, creating a sort of antibody-antigen-antibody sandwich (hence the name of the assay). Excitation light is then sent through the waveguide, exciting the fluorescent dye bound to the secondary antibody, indicating the presence of the target compound.
I remember learning about RI's Raptor sensor during my coursework early in my graduate studies, so it has been around for quite some time and has become a bit of a mainstay. Certainly if you'd like to learn more about either of these systems, you can check out Research International's website and read up about them. Besides plenty of reading material, there are some really nice diagrams on how the sandwich immunoassay works, as well as diagrams showing the inner workings of a fully automated biosensor. They also have some other cool detection and sampling platforms that are worth checking out if you're interested in those types of things.
And now for some news.
As if CERN hadn't made headlines enough lately (see Large Hadron Collider), they made big news last week when they announced and published the results of their recent studies on antimatter. One of two research groups working on parallel studies was able to effectively create atoms of antihydrogen, hold them in an electromagnetic field, and keep them there for nearly 200 milliseconds. Although this is a big deal, the end goal is to create and trap many more antihydrogens and to hold them for much longer periods of time so that the properties of the trapped antimatter can be thoroughly studied and examined. And so this study is a major step forward in regard to the overall objective.
And there you have it, folks. A blog update with actual substance. Been a while.